“An ounce of prevention is worth a pound of cure” – Benjamin Franklin
Any building owner that has had a coatings failure on their structure can relate to this quote. Seaside and coastal areas are beautiful locations for commercial and residential buildings, but also pose the greatest challenges in protecting exterior-facing architectural aluminum products from corrosion. Without proper precautions and finishes, corrosion to these aluminum components can damage the building envelope’s structural integrity, leading to systemic failure.
A painted coating or anodize finish meeting a project’s required performance and long-term durability can successfully be selected by carefully considering the building’s location, function, desired visual appearance and environmental attributes.
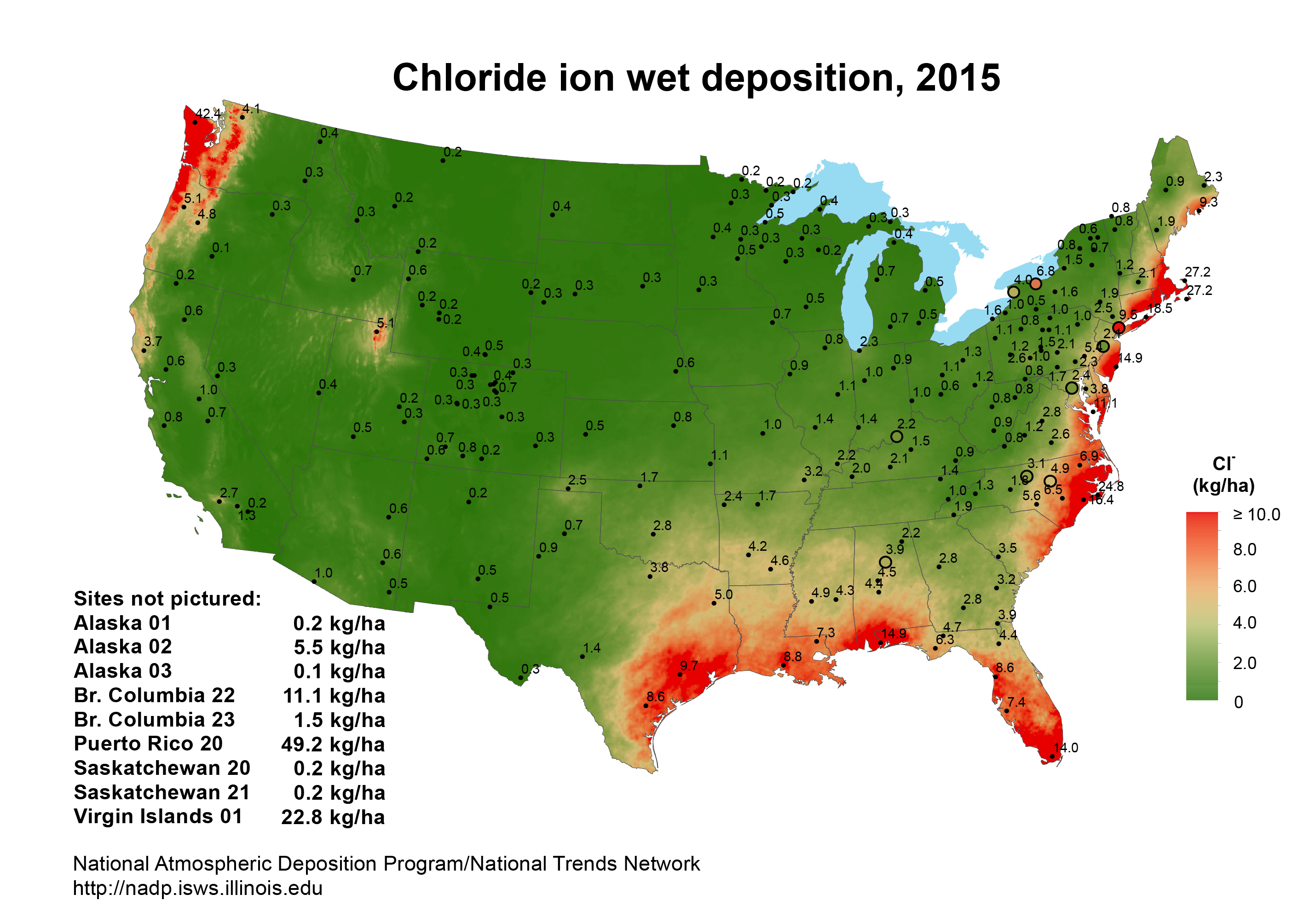 One of the most aggressive outdoor environments for aluminum is the seacoast as illustrated in this map, which compares wet chloride ion concentrations throughout the United States. Note that duration of wetness, temperature and frequency of temperature cycles are likely to be relatively higher in many coastal applications. Of the seacoasts, Florida’s coastal regions represent one of the most corrosive atmospheric environments in the continental U.S., which is why a testing site was established at the Kennedy Space Center, Cape Canaveral in the 1960s. Over the years, thousands of coated test panels have been evaluated in this high salt, high humidity, high UV radiation, Florida seacoast environment.
One of the most aggressive outdoor environments for aluminum is the seacoast as illustrated in this map, which compares wet chloride ion concentrations throughout the United States. Note that duration of wetness, temperature and frequency of temperature cycles are likely to be relatively higher in many coastal applications. Of the seacoasts, Florida’s coastal regions represent one of the most corrosive atmospheric environments in the continental U.S., which is why a testing site was established at the Kennedy Space Center, Cape Canaveral in the 1960s. Over the years, thousands of coated test panels have been evaluated in this high salt, high humidity, high UV radiation, Florida seacoast environment.
 The primary variables affecting corrosion rates near the coast are the salt concentration in the air, the duration of wetness of the metal surface, the temperature and the level of other atmospheric pollutants. Several environmental factors control these variables, including distance to the ocean, elevation, wind direction, wave action, rainfall, humidity, the degree of shelter and the level of industrial air pollution.
The primary variables affecting corrosion rates near the coast are the salt concentration in the air, the duration of wetness of the metal surface, the temperature and the level of other atmospheric pollutants. Several environmental factors control these variables, including distance to the ocean, elevation, wind direction, wave action, rainfall, humidity, the degree of shelter and the level of industrial air pollution.
A corrosive environment can consist of many different corrosive elements, including the most common—salt. Not all corrosive pollutants are found in a single corrosive environment; however a corrosive environment rarely contains only one type of corrosive. The more severe the atmosphere, the more critical a proper protective coating becomes.
Corrosion to aluminum can be defined as the deterioration of a substrate because of a reaction with its environment. An electrolyte must be present for corrosion to occur. An electrolyte is typically a water solution that can conduct an electric current because it contains salts, such as chlorides. The water could come from rain, condensation of humidity or fog.
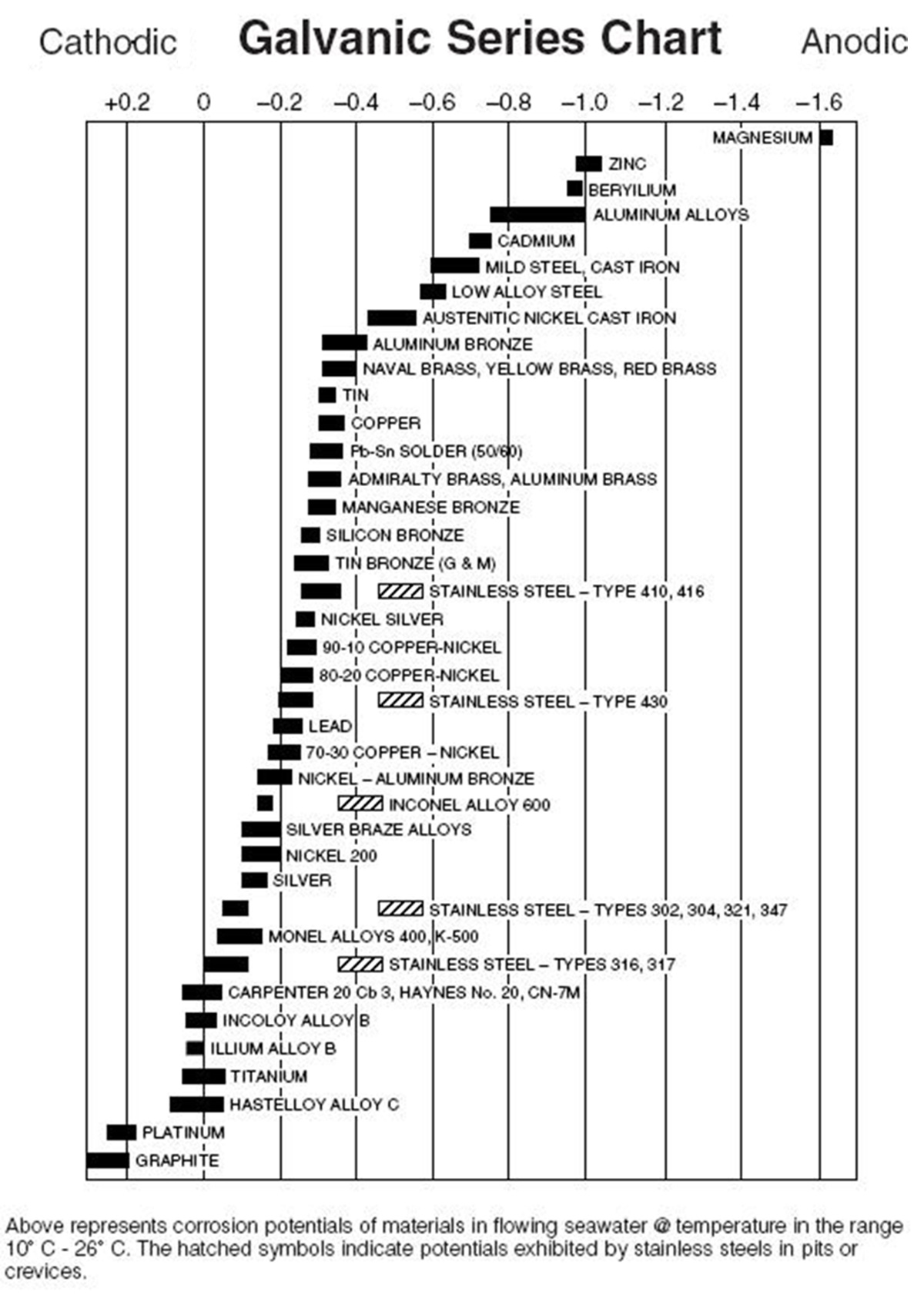 The are several forms of corrosion that can occur to metal, however the most common form associated with coatings’ protection of aluminum on buildings is atmospheric and galvanic corrosion. Galvanic corrosion is a real and common concern for most metals in coastal applications. In such environments, all it takes is contact between two dissimilar metals to initiate a galvanic reaction. The severity of corrosion largely depends on where the two metals in contact are in the galvanic series, contact area and the anode-to-cathode ratio. The further apart the materials are located on the galvanic series chart, the more likely the one on the anodic end corrodes when they are both wetted in any liquid considered to be an electrolyte.
The are several forms of corrosion that can occur to metal, however the most common form associated with coatings’ protection of aluminum on buildings is atmospheric and galvanic corrosion. Galvanic corrosion is a real and common concern for most metals in coastal applications. In such environments, all it takes is contact between two dissimilar metals to initiate a galvanic reaction. The severity of corrosion largely depends on where the two metals in contact are in the galvanic series, contact area and the anode-to-cathode ratio. The further apart the materials are located on the galvanic series chart, the more likely the one on the anodic end corrodes when they are both wetted in any liquid considered to be an electrolyte.
As a defensive measure, aluminum should be pretreated, whenever possible, with chrome phosphate. This should be followed by a primer, and then by a high-performance fluoropolymer paint or anodized in a Class I Architectural finish. After finishing, the aluminum should be isolated from other metals with non-absorbent, non-conductive, insulate or polymer sleeves and washers. Neoprene washers, roofing felt, paint or other bituminous coatings are effective barriers in exterior architectural products.
Durable Finishes
Painted coatings and anodized finishes are among the most durable finishes for exterior-facing architectural aluminum products. Along with long-lasting performance, surface appearance is an important characteristic of aluminum used for architectural applications.
As a prominent part of the buildings’ exterior, the coated aluminum adds color and design to the project. These coatings also protect the building from unsympathetic surroundings. When selecting a coating to stand up to harsh corrosive environments, one should specify either:
- The highest-performing organic paint coating that meets AAMA 2605-17, Voluntary Specification, Performance Requirements and Test Procedures for Superior Performing Organic Coatings on Aluminum Extrusions and Panels; or
- A Class I anodize coating that meets AAMA 611-14, Voluntary Specification for Anodized Architectural Aluminum.
These two options from the American Architectural Manufacturers Association (AAMA) continue to set the highest standard for architectural coatings, especially in a coastal or a highly corrosive environment.
Paint
High-performance 70 percent fluoropolymer coatings (often referred to by the brand name, Kynar) offer the capability to select nearly any conceivable color or combination of colors, while shielding the building against weathering, pollution and aging. Fluoropolymers that are used as binders in coatings include polytetrafluoroethylene (PTFE, or Teflon), fluorinated ethylene propylene (FEP), ethylene tetrafluoroethylene copolymer (ETFE),  polyvinylfluroide (PVF), polyvinylidene fluoride (PVDF), and fluoroethylene vinyl ether (FEVE). PVDF and FEVE are the most widely used in architectural systems. Arkema (Kynar) and Solvay Solexis (Hylar) are the two key manufacturers of PVDF.
polyvinylfluroide (PVF), polyvinylidene fluoride (PVDF), and fluoroethylene vinyl ether (FEVE). PVDF and FEVE are the most widely used in architectural systems. Arkema (Kynar) and Solvay Solexis (Hylar) are the two key manufacturers of PVDF.
The carbon-fluorine bond, used in 70 percent PVDF coating is one of the strongest known. These paint coatings have the ability to withstand enduring and intense UV radiation, attributed to long-term color- and gloss-retention, and chalk-resistance.
The first, and one of the most important, defenses against a paint failure is proper pretreatment of the aluminum. Without proper pretreatment, premature failure of the finish is almost guaranteed. Paint systems are designed to be applied over clean metal that has been properly pretreated. Pretreatment of the aluminum building components to be used in a severe corrosive or coastal environment is crucial.
The most time-tested, proven pretreatment system for architectural aluminum products is a chrome phosphate conversion coating. This process conforms to Type B, Method 5 of ASTM D1730-09(2014), Standard Practices for Preparation of Aluminum and Aluminum-Alloy Surfaces for Painting and ASTM D5723 - 95(2015) Standard Practice for Determination of Chromium Treatment Weight on Metal Substrates by X-Ray Fluorescence, as required by AAMA 2605-17.
Offering the longest lifecycle and true sustainability, chrome phosphate conversion coatings continue to be recognized by coating manufacturers as one of the most effective, robust pretreatments for aluminum. As a result, products installed along the seacoast and in other harsh industrial environments may not be warranted—or the warranty length and coverage could be compromised—when a chrome-free pretreatment system is employed.
After the aluminum has been properly pretreated, a primer coat is applied before the paint coating application. The paint coating is then sprayed to meet the AAMA 2605-17 specification of 1.2 mil (30 microns) total dry-film thickness. These highest-performing 70 percent fluoropolymer coatings are required to perform to rigorous testing performance standards, including more than 4,000 hours of salt spray, and heat- and humidity-resistance.
In addition, they must meet a minimum of 10 years on the South Florida testing site for film integrity, color retention, chalk resistance, gloss retention and erosion resistance. The AAMA 2605-17 document does note that in highly corrosive and high humidity environments such as, but not limited to, seacoast or industrial environments, coating performance may be diminished.
Anodize
When extreme hardness is required for aluminum building components, such as in high-traffic areas like entranceways and railings, an AAMA 611-14 anodized aluminum finish should be specified. The hardness of anodized aluminum rivals that of the diamond. (On the Moh scale of hardness, a 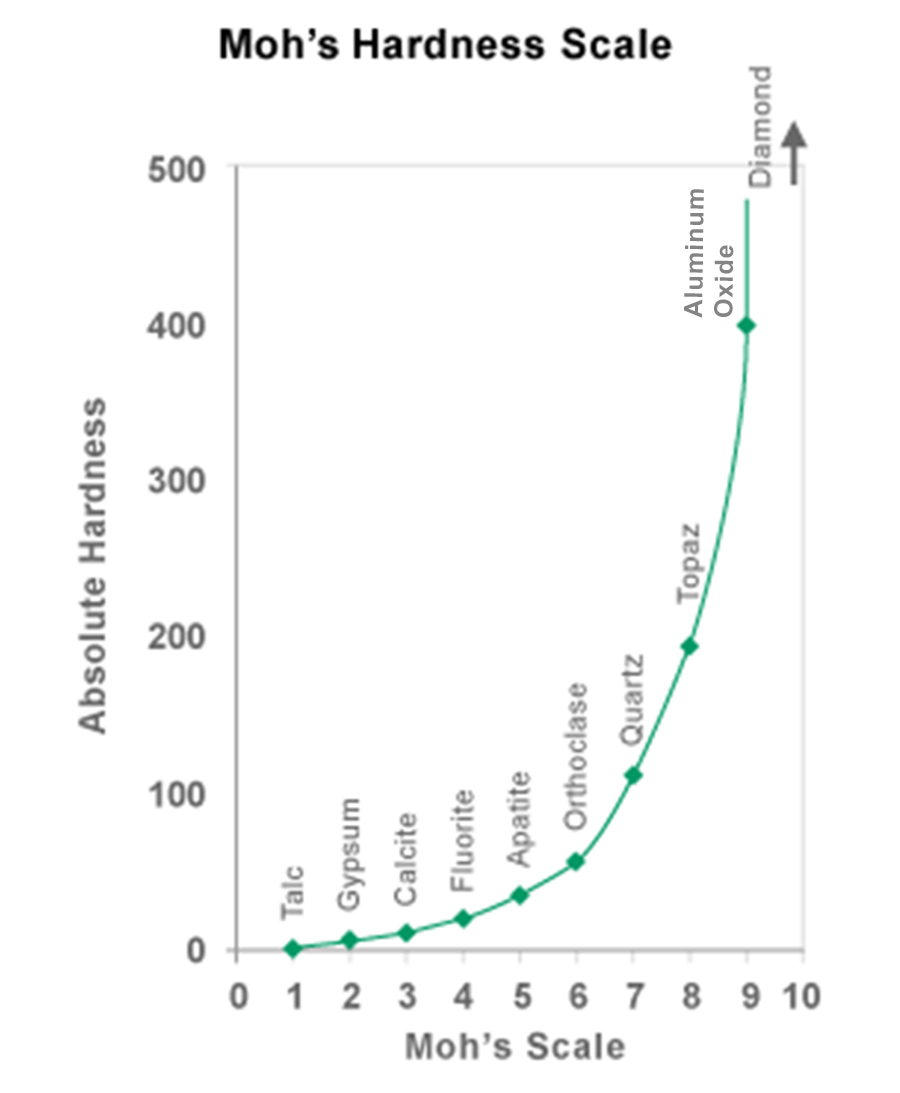 diamond is 10 and anodized aluminum is 9.)
diamond is 10 and anodized aluminum is 9.)
First used on an industrial scale in 1923 to protect age-hardenable aluminum alloy seaplane parts from corrosion, anodize was the first finishing technology developed for architectural applications. The Empire State Building, completed in 1931, contains aluminum window spandrels finished with a dark gray “deplated” finish. The term deplated appears in books and references from the 1930s, but disappears by the 1940s as it fell under the process of anodize. The anodize process provides corrosion protection, abrasion resistance and translucent color to aluminum surfaces. Architectural anodize is specified for its natural beauty, but also for its long-life and low maintenance. It provides excellent wear and abrasion resistance with minimal upkeep in most environments and resists the ravages of time, temperature, corrosion, humidity and warping.
High-performance anodize Class I coatings used in exterior applications require a minimum oxide coating thickness of 0.7 mils (18 microns). These architectural anodized aluminum coatings should meet the stringent guidelines and rigorous performance standards of AAMA 611-14, Class I specification including 3,000 hours of salt spray and corrosion resistance, plus a minimum of 10 years color retention and weathering on the South Florida on-fence testing site.
Copper anodize is available for architectural aluminum building products for those that want the rich look of copper without the design issues of dissimilar metals or copper runoff. Rainwater from real copper roofing or flashing can quickly  damage anodized aluminum. Aluminum and copper are far apart in the galvanic series, therefore using them together in a coastal or corrosive environment is not good practice.
damage anodized aluminum. Aluminum and copper are far apart in the galvanic series, therefore using them together in a coastal or corrosive environment is not good practice.
The copper anodize process is a unique, consistent and repeatable process that involves using actual copper to color the aluminum while isolating the copper in the coating. The rich copper color that is observed is actual copper that will not patina and will remain stable to provide many years of service. This copper anodize coating meets the AAMA 611-14 Class I specification.
Cleaning and Maintenance
An increased level of salt and other atmospheric pollutants can have a negative effect on finish longevity in the absence of periodic maintenance.
Runoff from adjacent site materials must be considered in a corrosion prevention plan. For example, mortar, cement and even gypsum dust can accumulate as alkaline deposits on aluminum surfaces and must be promptly rinsed. This is especially true of mill finish or anodized surfaces. While somewhat more resistant to alkaline attack than anodized surfaces, high-performance paint finishes can be managed with fresh water rinses to remove such buildup.
AAMA 609 and 610-15, Cleaning and Maintenance Guide for Architecturally Finished Aluminum, and AAMA CW 10-15, Care and Handling of Architectural Aluminum from Shop to Site, offer general guidelines, precautions and cleaning recommendations.
Galvanic corrosion of architectural aluminum materials is a fact that must be recognized; and proper steps must be taken to minimize the potential for its occurrence. With these building considerations and preventive measures in place, finished architectural aluminum will retain its intended look and long life, while providing the desired performance in the harshest environments, including the highly corrosive seacoast. These qualities reduce the need to replace materials and components, conserve resources, optimize labor and save money.



